Activity Monitoring
-
actibelt
®


 Capturing and analysing human motion
Capturing and analysing human motion
The actibelt is a wearable device for assessment of physical activity in clinical
trials developed and produced in Munich/Germany since 2005.
The level of a patient's walking ability or impairment in the context of controlled
clinical trials is usually measured by using questionnaires. Common problems are
substantial, uncontrollable bias and high inter- and intra-rater variability.
Invalid outcome measures and/or invalid surrogate markers in a variety of chronic
disabling diseases are a well-known problem. Physical activity in general and
walking ability (distance, speed, quality) in particular play a major role as
potential patient-oriented outcome measures and confounding factors in a broad range
of diseases, multiple sclerosis, CAD and COPD being prominent examples. Insufficient
assessment of this important variable that may also be a therapeutic option in its
own right may lead to unnecessary noise and even bias in the data, thereby diluting
and/or diminishing a potential beneficial effect of the treatment.
- Records multi sensor data with up to 100Hz for up to two months
- High compliance, no user interaction
- Robust positioning close to the body center of mass
- One-stop technology provider for algorithms, hardware, data management and consulting
- Parameters of interest for EMA/FDA e.g. real world walking speed
- Privacy by design, GDPR compliant, following GCP
- Global service in clincial trials since 2007 in EU, USA, AUS, JPN, RUS, KOR, TWN, BLR, COL, ARG, CHL...
- Used by NASA, ESA, DLR and Roskosmos
The RCT3 is certified according to EMC and electrical safety. EMC compatibility has been demonstrated against FCC and IEC60601-1-2 requirements. Electrical safety has been tested against IEC62368-1 (CB schema) as a consumer device. In addition, both RCT2 and RCT3 are voluntarily certified by SGS US (NRTL) to ensure that the products are compliant with the national safety standards in the US and Canada.
- Pioneers in algorithm development - 20 years of experience in development and validation of biosignal analysis in a regulatory context.
- High compliance - Two ingredients make the actibelt the best choice fo clinical trials and result in high compliance: First, the long battery runtime of up to two months, requiring no user interaction and second, the unobstrusive design by hiding the sensor in a belt buckle.
- Simple integration in clinical trials - The actibelt tooling was designed to make the handling of the actibelt and its data as simple as possible for all users - patients, investigators, site personal.
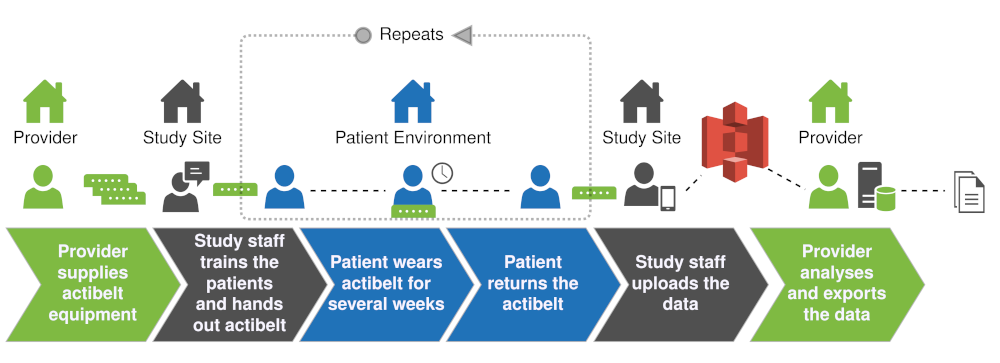
When the actibelt gets deployed in a clicinal trial we focus on making the
integration as lean as possible. We offer a cross-plattform software to configure
the actibelts and to upload the fully encrypted data into a cloud based storage
located in Germany.


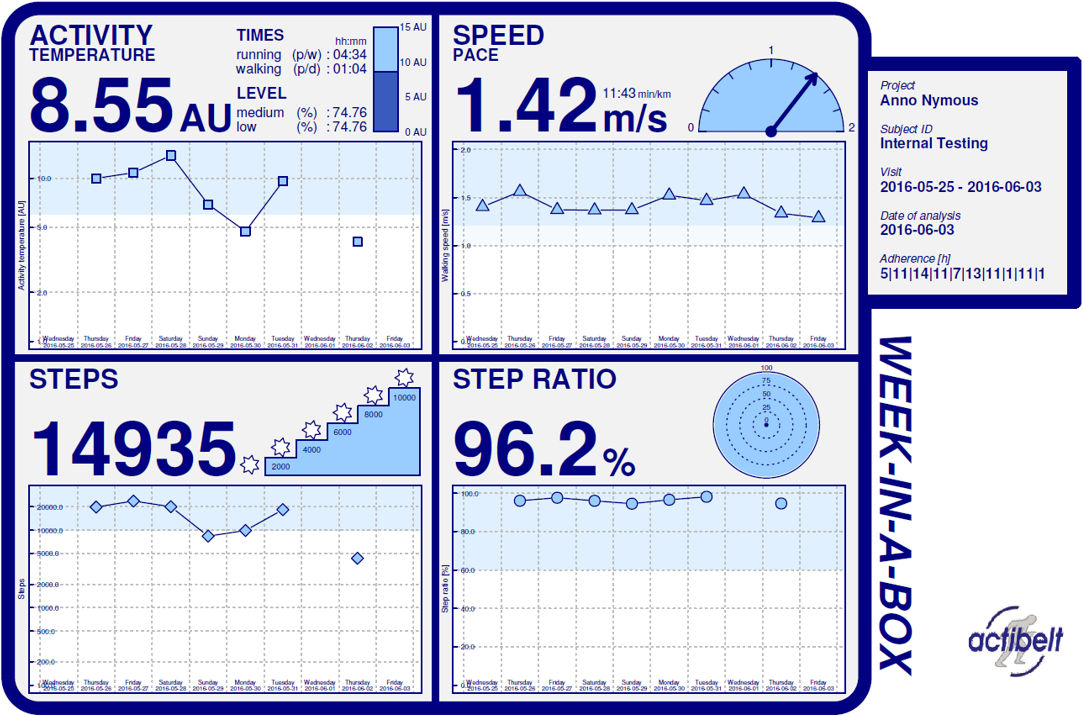
Activity parameters:
time belt worn per day, steps walking per day, walking step frequency per day, total
walking time per day, total distance travelled per day, mean velocity while
walking/real world gait speed per week, maximum coherent walking distance per day,
steps running per day, total running time per day, ratio of steps within sequences
of 50 or more steps per day, number of short distance periods, number of long
distance periods, activity temperature, activity level low, medium, high,
mediolateral body sway test (m/sec2)
Like the decoding of the human genome revealed insights into human health, our
measuring, studying and decoding of human movement, the "accelerome", reveals
insights into improving neurodegenerative diseases, falls, old-age immobility,
diabetes and obesity.

On a mission to the ISS in early 2024 ( https://www.esa.int/Newsroom/Press_Releases/ESA... ) the Swedish ESA astronaut Marcus Wandt was equipped with a wearable called "actibelt". https://www.axiomspace.com/mission-blog/ax3 . See also Wikipedia: https://en.wikipedia.org/wiki/Axiom_Mission_4

It is a multisensor device with accelerometers, gyroscopes, magnetometers and pressure sensors, embedded in a flexible belt buckle, to capture and analyze the astronaut's movements and exercises. This is a follow-up of a series of feasibility studies starting in 2007, with the goal determine if the actibelt can be used to quantify the activity profile and energy consumption of human participants while in a micro- or partial-gravity environment ( https://ntrs.nasa.gov/api/citations... , p137).

Exploration Medical Capability Group (ExMC)
Evaluation of the actiBelt® in a Microgravity Environment
June 19 - 22, 2007
The actibelt® platform is in use in various international multi-centre trials
and clinical-epidemiological studies in multiple sclerosis, CAD, osteoporosis,
lupus, osteoarthritis, Parkinson’s disease, depression, schizophrenia, fracture
heeling, COPD, sport & space science. Hundreds of patients, including children and
elderly people, have been equipped with the actibelt®, more than 25.000 hours of
recordings have been stored and analysed. The logistics allows for individualized
use up to "high-throughput accelerometry", e.g. up to 50 patients per day can be
equipped with the actibelt® per day per centre.

 Development of and usage of specialized simulation environments in
collaboration with TUM, DLR, ESA, e.g.
Development of and usage of specialized simulation environments in
collaboration with TUM, DLR, ESA, e.g.
Participants were pleasantly surprised at the comfort of the Actibelt, professing it to be one of the easiest devices to wear in the study. The belt required no interaction, which when combined with its perceived comfort, resulted in participants forgetting about the device. Because of this, participants expressed positive intentions to wear it within a clinical trial. However, some felt that they may get bored of it due to its lack of feedback. Furthermore, it may not be suitable with all clothing during the summer due to its obvious black colour. Insight Centre for Data Analytics, within University College Dublin (2018)









