Abstract
"Cardiotocography (CTG), introduced in the 1960s, was initially expected to prevent hypoxia-related deaths and neurological injuries. However, more than five decades later, evidence supporting the evidence of intrapartum CTG in preventing neonatal and long-term childhood morbidity and mortality remains inconclusive. At the same time, shortcomings in CTG interpretation have been recognised as important contributory factors to rising caesarean section rates and missed opportunities for timely interventions. An important limitation is its high false-positive rate and poor specificity, which undermines reliably identifying foetuses at risk of hypoxia-related injuries. These shortcomings are compounded by the technology's significant intra- and interobserver variability, as well as the subjective and complex nature of fetal heart rate interpretation. However, human factors and other environmental factors are equally significant. [...]"
doi.org/10.1111/1471-0528.18097
11 March 2025 08:30 - 16:00 CET | HYBRID
TranslaTUM - Central Institute for Translational Cancer Research
Einsteinstraße 25 (Building 522)
81675 Munich, Germany
Register until February 28th, 2025
humanmotioninstitute.de/register
Additional Contributors:
Gaya (Imperial College London): "Elevating Stroke Care: Wearable Devices for Behavioural Insights"
Bala (Imperial College London): "A wearable motion capture suit and machine learning predict disease progression in Friedreich’s ataxia" and "Wearable full-body motion tracking of activities of daily living predicts disease trajectory in Duchenne muscular dystrophy"
Dao (Imperial College London): "BehaviourGPT: Large Behaviour Models to measure health state in neurological diseases"
Sinead (Imperial College) : "Movement as a New Vital Sign and Early Warning Score of Deterioration"
Chaiyawan Auepanwiriyakul (University Bayreuth) : "Data-Driven Objective Markers for In-Ward Monitoring"
Lena Patricia Nieper (University Bayreuth) : "A Living Lab Paradigm for Real-World Neuroscience"
Christian Hieronimi (myoncare)
You can download the photos and videos at:
syncandshare.lrz.de/getlink/fiUhjsgUNeAJtbcwaRFMpc
"The Healthcare Hackathon gives you the chance to work with other creative minds over two days to develop innovative and progressive solutions that will take our industry forward. You can apply for challenges from the areas of women's health, pharmaceuticals, prevention, DiGAs and DiPAs, digital care and patient education or with your own personal challenge.
We cordially invite IT experts, nursing staff, doctors, trainees, students and anyone who wants to make a difference in the healthcare sector to the Healthcare Hackathon Bavaria. Let's come together in Erlangen and Munich to turn innovative ideas into real solutions or products."
bayern-innovativ.de/en/event/healthcare-hackathon-...
On a mission to the ISS in early 2024 the Swedish ESA astronaut Marcus Wandt was equipped with a wearable called "actibelt".
esa.int/Newsroom/Press_Releases/ESA_astronaut_Marc...
It is a multisensor device with accelerometers, gyroscopes, magnetometers and pressure sensors, embedded in a flexible belt buckle, to capture and analyze the astronaut's movements and exercises. This is a follow-up of a series of feasibility studies starting in 2007, with the goal determine if the actibelt can be used to quantify the activity profile and energy consumption of human participants while in a micro- or partial-gravity environment.
ntrs.nasa.gov/api/citations/20080015863/downloads/... (p137).
Date: TUESDAY, 6TH OF AUGUST, 2024, Time:9:00-16:10
Location: At the auditorium of TranslaTUM Central Institute for Translational Cancer Research, Einsteinstraße 25 (Building522), 81675Munich, Germany
You download presentations, documentation and videos at:
syncandshare.lrz.de/getlink/fiWmfYRvDrSQfRBNsG2qfM
* Welcome/Overview, Dr. Martin Daumer
* Patient Safety-aware Telemedical Interface, Nihat Erdebil, Minghui Li, Nathalie Schneider, Tasnia Shaikh
* DysphagAI Enhancing High-Resolution Manometry (HRM) Examinations through AI-based Expert-Level System Skander Znaidi, Oumaima Ben Hadj Khaled, Walid Ghazouani, Mihyar Ouni
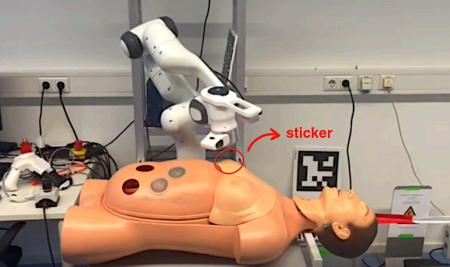
* Semiautonomous Telerobotic Auscultation with Surface Normal Estimation
Delun Zhang, Jeff Lin, Zikang Liu, Yuya Yuan
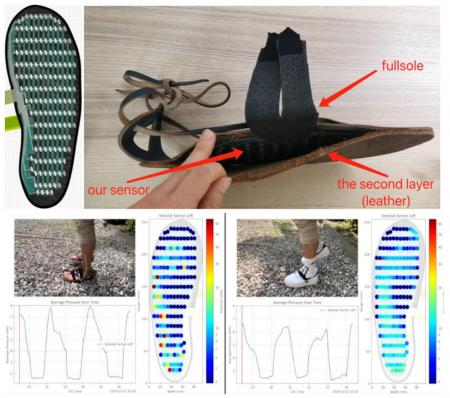
* Footsole Pressure sensing, Kai Burian, Chutong Ren, Arved Strauch
* Real-time Dynamic Endoscopic Scene Reconstruction with 4D Gaussian Splatting, Wenbo Ji
* 3D Foot Scanner, Weiqing Lou, Chao Li, Chenyue Tang, Linfei Mao, Siyi Ren, Ruicong Zhang, Sichun Zhu, Shiwen Cao, Zihao Chen, Zenan Zhao
* MedSense: Medical Video Retrieval System, Junzhe Hu, Sitong Liu, Zihan Xu, Zongxie Chen
* MITI x TUM: Prediction of post-surgical complications using artificial intelligence, Patrick Schneider, Tilmann Bruns, Johannes Weig, Michael Baumgärtner
* Somatization and AI: Towards More Accurate Diagnosis of Medically Unexplained Symptoms Marina Romero del Hombrebueno, Lucia Zorroza, Shruti Nair, Karahan Yilmazer